

The lower part of the furnace is kept at a much higher temperature than the top. Actually, the reduction of the iron oxides happens at different temperatures. Here the ore mixes with coke and limestone in the furnace. 2) Extraction of Iron Extraction of iron from its oxide is done in a blast furnace. Since aluminium oxide is more stable it is used in the extraction of chromium by a thermite process. This essentially means Aluminium can be used as a reducing agent for oxides of all the metals that lie above it in the graph. The diagram gives us no representation of this scenario Uses of Ellingham Diagram 1) Alumino Thermic Process The Ellingham curve on the graph actually lies lower than most of the other metals such as iron. Say for example more than one oxide is possible. Also, it does not provide complete information about the oxides and their formations. Limitations of Ellingham Diagram It does not consider the kinetics of the reactions. And as a result, this curve will go downwards. 2C (s)+ O2 (g) → 2CO (g): Here one mole of gas is giving you two moles of gas as products. So there will be no slope, it is completely horizontal. So here one molecule of gas is resulting in one molecule of gas. Let us take a look at few such examples C(s) + O2 (g) → CO2 (g): Entropy of solids is negligible. Hence as the temperature increases, the value of TΔS will also increase, and the slope of the reaction goes upwards Exceptions to Ellingham Diagram There are cases when the entropy is not negative, and the slope will not be upwards. Also since in the reaction (as seen above), we are going from the gaseous state to the solid state ΔS is also negative. We will plot the temperature on the Y-axis and the ΔG on the X axis Metals that have curves at the bottom of the diagram reduce the metals found more towards the top The reaction of metal with air can be generally represented as M (s) + O2 (g) → MO (s) Now when reducing metal oxides the ΔH is almost always negative (exothermic) reaction. However, there is a condition here, that a phase change should not occur. As you know the ΔH (enthalpy) is not affected by the temperature Even ΔS that is the entropy is unaffected by the temperature. The slope of the curve is the entropy and the intercept represents the enthalpy.
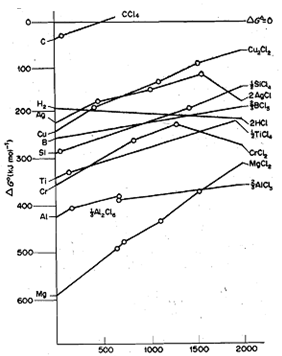
Let us take a look at some important properties of the Ellingham Diagram Here ΔG is plotted in relation to the temperature. This helps us to find the most suitable reducing agent when we reduce oxides to give us pure metals. The species amounts must be balanced to contain exactly the same amount of the main element, such as oxygen in oxides and sulfur in sulfides. These diagrams must contain only the same type of substances, such as oxides, sulfides, chlorides, etc. In metallurgy, we make use of the Ellingham diagram to plot the reduction process equations. The DG (Ellingham) diagrams show the relative stability of various oxides, sulfates, chlorides etc. It is basically a graphical representation of Gibbs Energy Flow. So having the lines not meet exactly could actually provide an additional bit of information behind the data.Metallurgical engineering - An Ellingham diagram shows the relation between temperature and the stability of a compound. If all three experiments had a similar amount of experimental error, then the deviation from concurrency in the diagram would provide a rough measure of this error.
ELLINGHAM DIAGRAMS FREE
In the case of carbon oxidation, if we draw on measured thermodynamic data for oxidizing $\ce$, in which case the three independently determined free energy lines would also not match up. Whether the lines are exactly concurrent depends on how the data were obtained.


 0 kommentar(er)
0 kommentar(er)
